ABOUT US
Who We Are?
Founded in 1994, Rusan Pharma is a fully integrated global pharmaceutical company specializing in the treatment of addiction and pain management. Rusan’s manufacturing company for active pharmaceutical ingredients (API) and finished pharmaceutical formulations, live up to some of the highest standards of good manufacturing practices (GMP) and are approved by Health Canada.
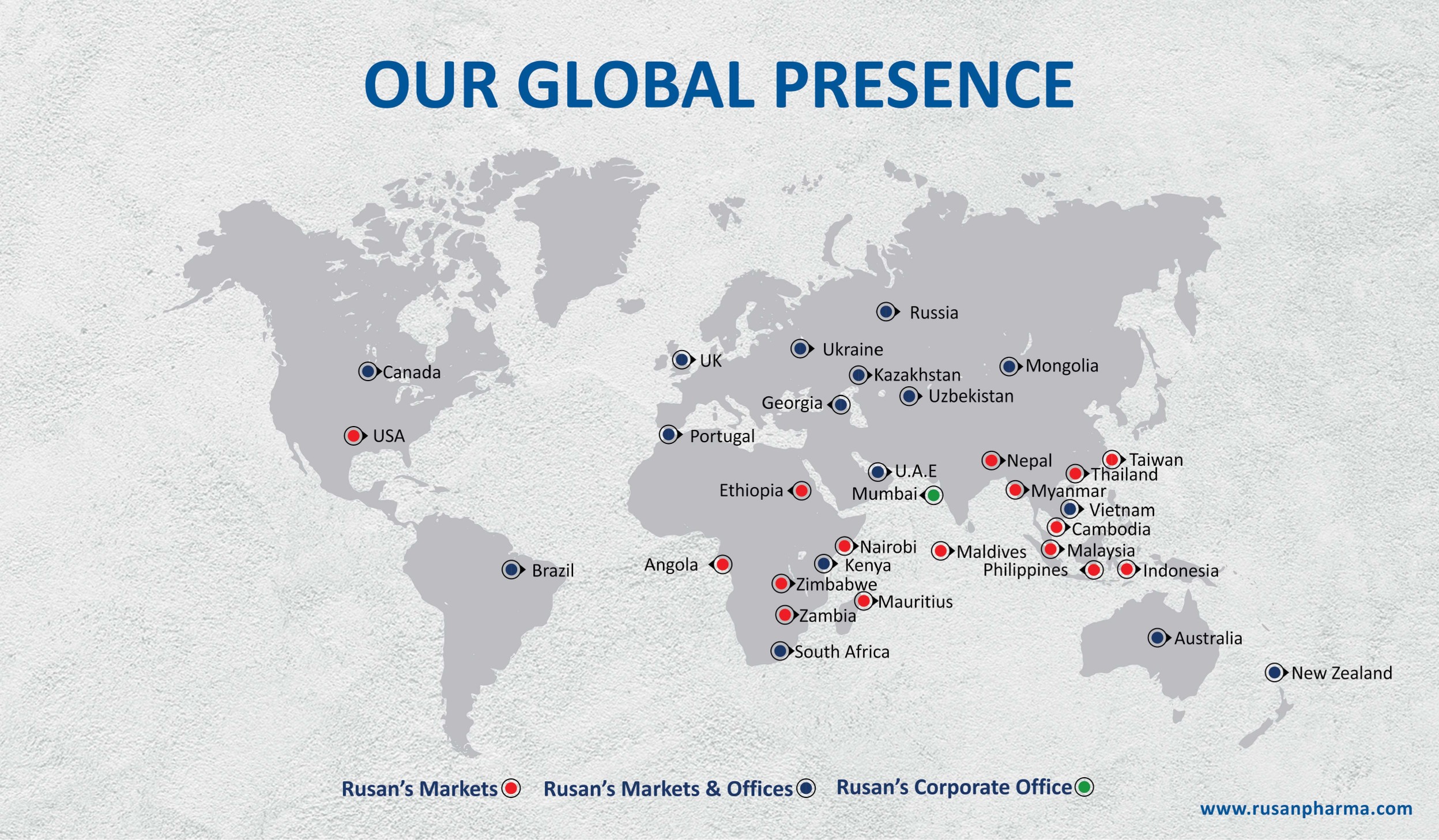
Rusan Pharma Canada Inc. (RPCI) is the Canadian subsidiary of Rusan Pharma Ltd. (India), a generic pharmaceutical company located in Montreal, Québec. With a strong product development pipeline and strategic partnerships, we pride ourselves in delivering high quality and affordable generic pharmaceutical medicines to the Canadian market. Through product differentiation, best-in-class supply and excellent service, we strive to help families across Canada live longer, healthier and happier lives.
Our Mission
Rusan firmly believes that access to quality healthcare is a right, not a privilege. We will endeavour to ensure the availability of world class, innovative, quality medicines at affordable prices, across the globe and are committed to work towards a healthier & happier world.
Our Vision
To bring forward innovative, highly quality novel and generic medications at affordable prices for our consumers.
OUR LEADERS

Dr. Kunal Saxena
Managing Director, Rusan PharmaA technocrat with wide exposure to process development, synthetic chemistry & commercialization of bulk drugs. A practicing technocrat with vast experience in research & development. Specialised in computer-aided drug design and new chemical entity synthesis including chiral synthesis. Dr. Kunal Saxena has done his Ph.D. in Pharmacology from the University of Bristol (UK), M.Sc. in Chemistry from Moscow State University (Moscow, Russia) and B.Sc. in Biochemistry from the University of Notre Dame (USA). Has done basic research in the area of opioid receptors and developed several novel non-opioid compounds that help reduce side effects and tolerance to opioid analgesics.

Dr. Aakarsh Saxena
Director, Rusan PharmaDr. Aakarsh Saxena, based in Mumbai, is currently a Director at Rusan Pharma Ltd, bringing experience from previous roles at The University of Texas at Austin, The University of Edinburgh, William & Mary and Rusan Pharma Ltd. Dr. Aakarsh Saxena holds a 2008 - 2013 Doctor of Philosophy (Ph.D.) from The University of Edinburgh. With a robust skill set that includes Chemistry, Organic Chemistry, Catalysis, Asymmetric Catalysis, NMR Spectroscopy and more, Dr. Aakarsh Saxena contributes valuable insights to the industry.

Dr. Navin Saxena
Chairman, Rusan PharmaPh.D. in Synthetic Organic Chemistry from Moscow Friendship University. Dr. Navin Saxena started his career in 1981 as a Research Officer and within a short span of 10 years reached the level of Vice President and Head of Research. Dr. Navin Saxena is not only the youngest recipient of this award, but also the first Indian to receive this accolade. On 29th July 1997, Dr. Saxena in the capacity of being the Chairman of Rusan Pharma Ltd. was honoured with "THE INTERNATIONAL EXECLLENCE AWARD" by the Council for Small and Medium Exporters for excellence in exporting medicines. Dr. Saxena was also awarded the prestigious "UDYOG RATTAN AWARD" by the Institute for Economic Studies for his contribution in the field of industrial development in India.
API
API Active Pharmaceutical Ingredient (API)
| Sr. No. | API Name | GRADE | Approval status CEP/ DMF | CAS No. |
|---|---|---|---|---|
| 1 | Buprenorphine | EP/BP/USP | Rl-CEP 2015-387 - Rev 00 | 52485-79-7 |
| 2 | Buprenorphine Hydrochloride | Ph. Eur. / USP | R1-CEP 2017-228 - Rev 00 | 53152-21-9 |
| 3 | Fentanyl | Ph. Eur. / USP | Rl-CEP 2016-018 - Rev 00 | 437-38-7 |
| 4 | Fentanyl Citrate | Ph. Eur. / USP | R1-CEP 2016-023 - Rev 00 | 990-73-8 |
| 5 | Naltrexone Hydrochloride | Ph. Eur. / USP Ph. Eur. |
Rl-CEP 2016-053 - Rev 00 CADIFA: 25351.002633/2022-17 Rev 001 |
850808-02-5 |
| 6 | Methadone Hydrochloride | Ph. Eur. / USP | CEP 2017-277 - Rev 02 | 1095-90-5 |
| 7 | Bisoprolol Fumarate | Ph. Eur. / USP | R0-CEP 2020-095- Rev 00 | 104344-23-2 |
| 8 | Apomorphine Hydrochloride Hemihydrate | Ph. Eur. / USP | CEP 2021-135 - Rev 00 | 41372-20-7 |
| 9 | Naloxone Hydrochloride dihydrate | Ph. Eur. / USP | CEP 2022-086 - Rev 00 | 51481-60-8 |
| 10 | Diazepam | Ph. Eur. | CEP 2024-260 | 439-14-5 |
| 11 | Clonazepam | Ph. Eur. | CEP 2025-232 | 1622-61-3 |
| 12 | Eflornithine Hydrochloride monohydrate | Non-pharmacopeial | EMA approved EU/ASMF/00137 Active US DMF (№ 031780) |
96020-91-6 |
| 13 | Nalmefene Hydrochloride | Non-pharmacopeial | Active US DMF (№ 034367) | 1228646-72-7 |
| 14 | Nalbuphine Hydrochloride | Non-pharmacopeial | DMF Available | 23277-43-2 |
| 15 | Promedol Hydrochloride | Non-pharmacopeial (Russian pharmacopeia) |
DMF Available | 64-39-1 |
| 16 | n-Butyl Cyanoacrylate | Non-pharmacopeial | DMF Available | 6606-65-1 |
| 17 | Naltrexone | Non-pharmacopeial | DMF Available | 16590-41-3 |
| 18 | Sodium Oxybate | Non-pharmacopeial | DMF Available | 502-85-2 |
| 19 | Oxycodone Hydrochloride | Ph. Eur. | DMF Available | 124-90-3 |
| 20 | Naltrexone Hydrochloride (anhydrous) | Ph. Eur. | DMF Available | 16676-29-2 |
API Active Pharmaceutical Ingredient (API)
| Sr. No. | API Name | GRADE | CAS No. |
|---|---|---|---|
| 1 | Nalbuphine | Non-pharmacopeial | 20594-86-6 |
| 2 | Carbamazepine | Ph. Eur. | 298-46-4 |
| 3 | Dinalbuphine Sebacate | Non-pharmacopeial | 311768-81-7 |
| 4 | Naltrexone Decanoate | Non-pharmacopeial | 664354-91-0 |
| 5 | Remifentanil Hydrochloride | Ph. Eur. | 132539-07-2 |
| 6 | Sufentanyl | Ph. Eur. | 56030-54-7 |
| 7 | Sufentanyl Citrate | Ph. Eur. | 60561-17-3 |
| 8 | Midazolam | Ph. Eur. | 59467-70-8 |
| 9 | Dihydrocodeine hydrogen Tartrate | Ph. Eur. | 5965-13-9 |
| 10 | Hydromorphone Hydrochloride | Ph. Eur. | 71-68-1 |
| 11 | Hydrocodone Hydrogen Tartrate 2.5 Hydrate | Ph. Eur. | 125-29-1 |
| 12 | Oxymorphone Hydrochloride | USP | 357-07-3 |
| 13 | Pregabalin | Ph. Eur. | 148553-50-8 |
| 14 | Methyl Naltrexone Bromide | USP | 916055-92-0 |
| 15 | Tramadol Hydrochloride | Ph. Eur. | 36282-47-0 |
| 16 | Temazepam | Ph. Eur. | 846-50-4 |
| 17 | Nitrazepam | Ph. Eur. | 146-22-5 |
| 18 | Oxazepam | Ph. Eur. | 604-75-1 |
| 19 | Lorazepam | Ph. Eur. | 846-49-1 |
| 20 | Paliperidone base | USP | 144598-75-4 |
| 21 | Paliperidone palmitate | In-house | -- |
Finished Formulation
All Products
| Sr. No. | Product Name | Active Ingedients | Strength | Fill Size | Pack Size | Pack Format | Indication | Image | DIN | UPC | Patient Information Leaflet (PIL) | Product Monograph |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
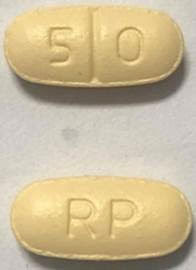
| 1 | RPC-NALTREXONE (Naltrexone Hydrochloride Tablets) |
Naltrexone Hydrochloride | 50 mg | N/A | 30 Tabs | Blister | Alcohol Dependence | View | 02558343 | click to view | click to view | |
| 2 | RPC-NALTREXONE (Naltrexone Hydrochloride Tablets) |
Naltrexone Hydrochloride | 50 mg | N/A | 50 Tabs | Blister | Alcohol Dependence | View | 02558343 | click to view | click to view | |
| 3 | RPC-NALTREXONE (Naltrexone Hydrochloride Tablets) |
Naltrexone Hydrochloride | 50 mg | N/A | 100 Tabs | Blister | Alcohol Dependence | View | 02558343 | click to view | click to view | |
| 4 | RPC-NALTREXONE (Naltrexone Hydrochloride Tablets) |
Naltrexone Hydrochloride | 50 mg | N/A | 30 Tabs | HDPE bottle | Alcohol Dependence | View | 02558343 | click to view | click to view | |
| 5 | RPC-NALTREXONE (Naltrexone Hydrochloride Tablets) |
Naltrexone Hydrochloride | 50 mg | N/A | 50 Tabs | HDPE bottle | Alcohol Dependence | View | 02558343 | click to view | click to view |
All Products
| Sr. No. | PRODUCT NAME | ACTIVE INGREDIENTS | STRENGTH | FILL VOLUME | PACK SIZE | PACK FORMAT | INDICATION |
|---|---|---|---|---|---|---|---|
| 1 | RPC – Methadone (no sugar, no colour, no flavour) | Methadone Hydrochloride | 10 mg / mL | 1000 mL | Bottle of 1 Litre | HDPE bottle | De-addiction |
| 2 | RPC – Methadone (cherry flavour, with sugar) | Methadone Hydrochloride | 10 mg / mL | 500 mL | Bottle of 500 mL | HDPE bottle | De-addiction |
| 3 | RPC – Methadone (cherry flavour, with sugar) | Methadone Hydrochloride | 10 mg / mL | 1000 mL | Bottle of 1 Litre | HDPE bottle | De-addiction |
| 4 | RPC – Buprenorphine + Naloxone sublingual tablets | Buprenorphine Hydrochloride + Naloxone Hydrochloride | 8 mg + 2mg | - | 30 tablets | HDPE bottle | De-addiction |
| 5 | RPC – Buprenorphine + Naloxone sublingual tablets | Buprenorphine Hydrochloride + Naloxone Hydrochloride | 8 mg + 2mg | - | 7 tablets | Alu-Alu blister | De-addiction |
| 6 | RPC – Buprenorphine + Naloxone sublingual tablets | Buprenorphine Hydrochloride + Naloxone Hydrochloride | 2 mg + 0.5 mg | - | 30 tablets | HDPE bottle | De-addiction |
| 7 | RPC – Buprenorphine + Naloxone sublingual tablets | Buprenorphine Hydrochloride + Naloxone Hydrochloride | 2 mg + 0.5 mg | - | 7 tablets | Alu-Alu blister | De-addiction |
| 8 | RPC-Nalbuphine Injection | Nalbuphine Hydrochloride Injection 10 mg / mL | 10 mg / mL | 1 mL | Pack of 10 ampoules | 1 mL USP Type I ampoules | Pain Management |
| 9 | RPC – Haloperidol Injection | Haloperidol Injection | 5 mg / mL | 1 mL | Pack of 5 ampoules | 1 mL USP Type I Amber ampoules | Antipsychotic |
| 10 | RPC – Ephedrine Injection | Ephedrine Injection | 50 mg / mL | 1 mL | Pack of 10 ampoules | 1 mL USP Type I Amber ampoule | Used in clinically significant hypotension |
Our Facilities
| Dosages Form |
|---|
|
Tablets |
|
Capsule |
|
Powder in Sachet |
|
Lyo Injectable (Vial / Bulk Powder) |
|
Small Volume Parenteral - Vial |
|
Small Volume Parenteral (Injectable) - Ampoule |
|
Cream & Ointment |
|
Beta-Lactam Capsule |
|
Beta-Lactam Dry Syrup |
Approved By










| Dosages Form |
|---|
|
Tablet |
|
Capsule |
|
Oral Liquid - Solution / Suspension (30 ml to 1000 ml) |
|
Transdermal Patch |
|
Small Volume Parenteral (Injectable) - Vial |
|
Small Volume Parenteral (Injectable) - Ampoule |
|
Small Volume Parenteral (Injectable) - Prefilled Syringe |
Approved By












R&D
Navin Saxena Research & Technology Pvt. Ltd. (NSRT)
NSRT, an affiliate of Rusan pharma, is a state-of-the-art research centre in the Kandla Special Economic Zone (KASEZ; Gujarat, India). With a rich experience of more than 25 years, in pharmaceutical development & manufacturing, NSRT is capable of undertaking cutting-edge research. Our research team comprises of versatile and specialized pool of scientists in the field of computer aided drug design, development and manufacturing of API and novel drug delivery systems.
NSRT is capable of undertaking R&D projects for custom API synthesis and development of finished formulations and in partnership with Rusan Pharma, undertake commercial production for the Canadian market.

Quantys Clinical Pvt Ltd. (QCPL)
QCPL is a 104 bed clinical research organization (CRO) with five independent clinics with collapsible partitions, enabling QCPL to conduct large, mixed population and multiple studies at the same time. QCPL's trained & dedicated staff brings in considerable experience in conducting Pharmacokinetic (PK) and Clinical trial studies for various finished formulations.
QCPL's partnerships with Investigators, Institution, NGO’s, Hospitals and Clinics, enable it to recruit not just healthy volunteers but ‘Special Volunteer Groups’ to cater to the specific study requirements.
QCPL can provide PK and clinical trial study services to Canadian Pharmaceutical companies looking to conduct such studies.

Product Pipeline
Innovation is the cornerstone of Rusan’s vision & mission. Our rich development portfolio focuses in the area of addiction treatment, pain management and Parkinson’s. To partner with Rusan on some of our novel & generic transdermal patch products connect with us: formulation.enquiry@rusanpharma.com
| Indication | Program / Regulatory Pathway | Preclinical | Phase-I | Phase-II | Phase-III | Approval |
|---|---|---|---|---|---|---|
| Alcohol Use Disorder | Antagonist Prodrug Sustained Release Injection (Monthly) |
|
||||
| Opioid Use Disorder | Partial Agonist Weekly & Monthly Sustained Release Injection |
|
||||
| Pain Management | Partial Agonist Monthly Sustained Release Injection |
|
||||
Generic Transdermal Patches & Oral Film
For exploring in-licensing opportunities for our transdermal patch portfolio for the Canadian market, please Email us on: formulation.enquiry@rusanpharma.com
| Generic Name | Remarks |
|---|---|
| Nicotine Patch | Under Development |
| Nicotine Oral Flim | Under Development |
| Buprenorphine + Naloxone Sublingual Flim | Under Development |
| Buprenorphine Patch | Under Development |
| Fentanyl Patcht | Under Development |
| Rivastigmine Patch | Under Development |
| Rotigitine Patch | Under Development |
| Lidocaine Patch | Under Development |
CAREERS
"Great Products are the brainchild and hard work of great people. Join a young, dynamic, fast-growing and inclusive company where you have the opportunity to aim high, fulfil your potential, and achieve great things!"